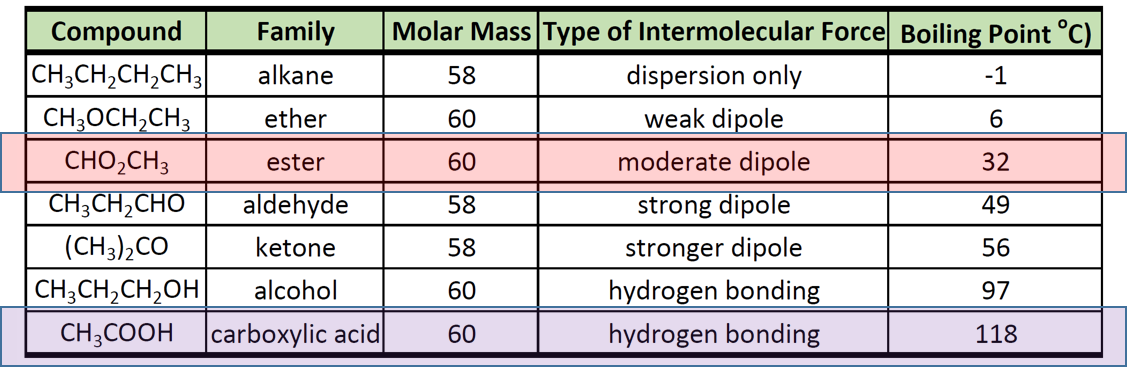
Boiling Point of Ester and Carboxylic Acid
The general molecular formula for dicarboxylic acids can be written as HO 2 CRCO 2 H where R can be aliphatic or aromatic. Acidic strength of aromatic acids The parent member of the family benzoic acid which is a weaker acid K a 63 x 10-5 than acid K a 177 x 10-5 but stronger than acetic acid.

Lesson Explainer Properties Of Esters Nagwa
Free carboxylic acid groups may.
. Some order of acidity are b Similarly K a. As with carboxylic acids two phosphoric acid molecules may combine with the loss of water to form a di phosphate ester also referred to as pyrophosphate. In general dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acidsDicarboxylic acids are also used in the preparation of.
Why do you wash the dichloromethane solution of your reductive amination product with sodium bicarbonate rather than dilute aqueous HCl. Carboxylic acid any of a class of organic compounds in which a carbon C atom is bonded to an oxygen O atom by a double bond and to a hydroxyl group OH by a single bond. Salts and esters of valeric acid are known.
However as phosphoric acid has further -OH functionalities triphosphates may also be formed. A Sodium bicarbonate is a good method of removing aldehydes from organic solventb The amine product will be protonated by acid and remain in the aqueous layer as a saltc Sodium bicarbonate transfers the amine starting. Cracking 1 C10H22 C2H4 C8H18 1 Step 2.
Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH 3 CH 2 3 COOHLike other low-molecular-weight carboxylic acids it has an unpleasant odorIt is found in the perennial flowering plant Valeriana officinalis from which it gets its nameIts primary use is in the synthesis of its esters. COOO-H is a weaker acid than acetic acid as acetate ion is stabilised by resonance. Ethyl methanoate or methyl ethanoate 1 correctly displayed ester linkage 1 fully correct displayed formula corresponding to C3H6O2 and matching named ester 1 3 7civ displayed formula of propanoic acid 1 propanoic acid 1 2 7d Step 1.
7ciii name of ester corresponding to C3H6O2. A fourth bond links the carbon atom to a hydrogen H atom or to some other univalent combining group. Its methyl ester does however.
A dicarboxylic acid is an organic compound containing two carboxyl functional groups COOH. The carboxyl COOH group is so-named because of the carbonyl group CO and hydroxyl group. Amide synthesis from carboxylic acid and any pst amine depends upon chain lenght of both compound and boiling point of both.

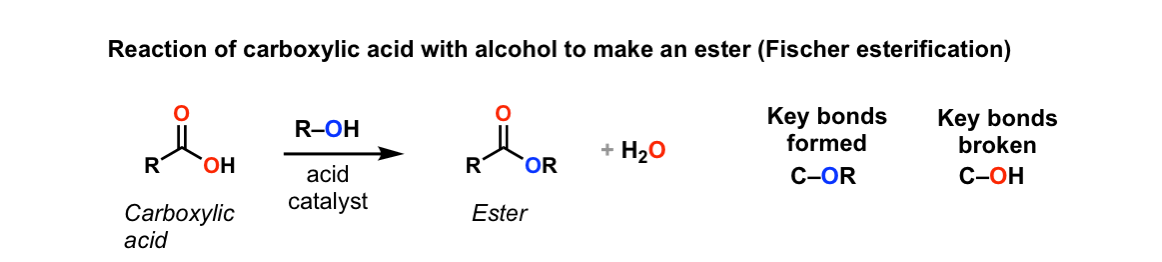
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry
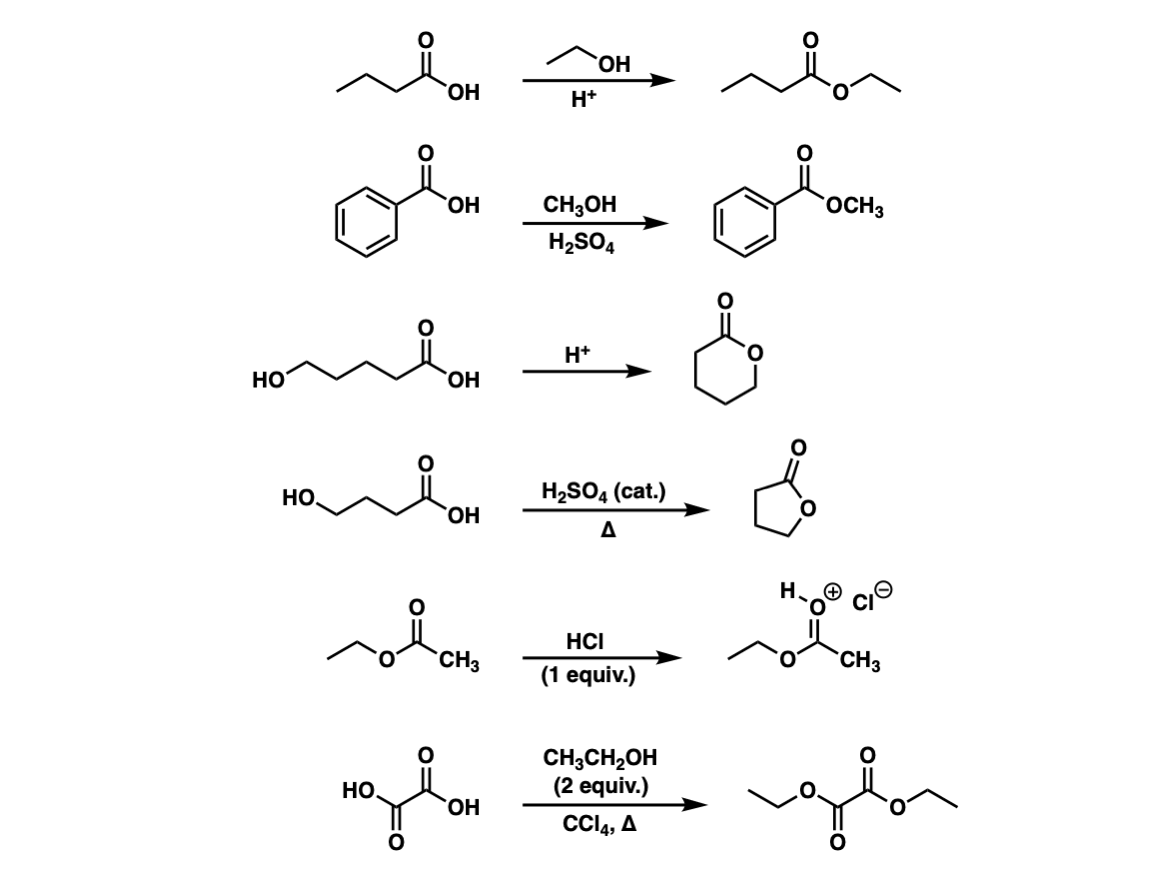
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry

Physical Properties Of Carboxylic Acids Youtube
0 Response to "Boiling Point of Ester and Carboxylic Acid"
Post a Comment